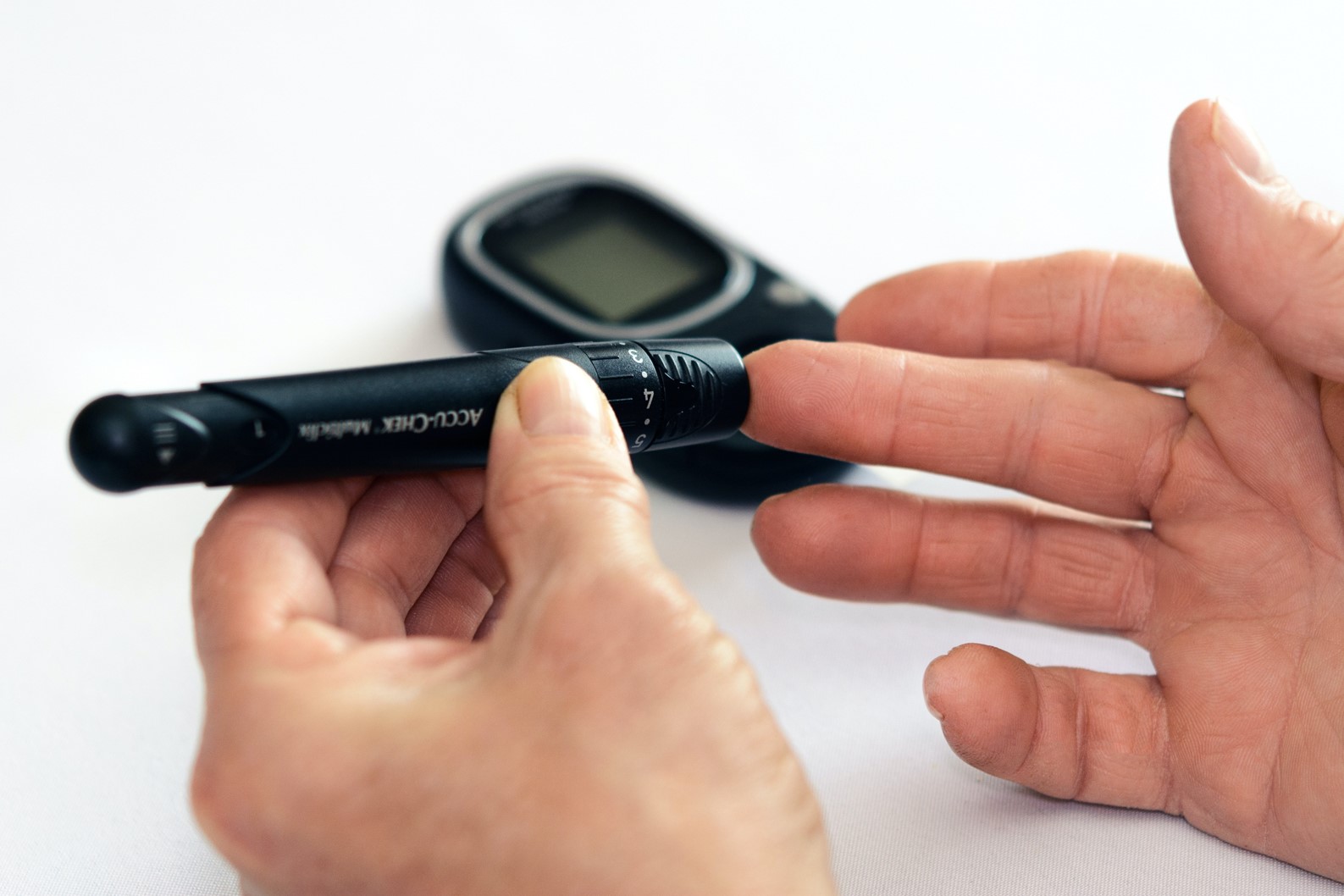
Diabetes Medication Demonstrates Potential for Sleep Apnea Treatment A groundbreaking study has shown promising results for a diabetes medication in resolving sleep apnea, a common sleep disorder that affects millions worldwide. The medication, semaglutide, is a glucagon-like peptide-1 (GLP-1) agonist used to treat type 2 diabetes. In a clinical trial, researchers discovered that semaglutide significantly improved sleep apnea symptoms in up to 52% of patients. Mechanism of Action Sleep apnea is caused by the relaxation of the muscles in the throat during sleep, which blocks the airway and leads to pauses in breathing. Semaglutide is believed to work on the central nervous system to stimulate the muscles responsible for maintaining airway patency during sleep. Impressive Results The study included 175 patients with both type 2 diabetes and sleep apnea. Participants were randomly assigned to receive either semaglutide or a placebo. After 68 weeks, the semaglutide group experienced a 52% reduction in the apnea-hypopnea index (AHI), a measure of the severity of sleep apnea. In contrast, the placebo group showed only a 16% reduction. Improved Sleep Quality Beyond its impact on AHI, semaglutide also improved other aspects of sleep quality. Patients reported reduced daytime sleepiness, better sleep efficiency, and increased rapid eye movement (REM) sleep, which is important for cognitive function and mood. FDA Approval Pathway The promising results of this study have sparked interest in the potential FDA approval of semaglutide for the treatment of sleep apnea. The manufacturer of the medication has not yet announced its plans for seeking FDA approval, but it is likely that further clinical trials and regulatory filings will be required. Conclusion The discovery that a diabetes medication can effectively resolve sleep apnea opens up exciting possibilities for treating this prevalent disorder. While FDA approval is still pending, the study’s findings suggest that semaglutide could become a valuable therapeutic option for millions of patients who suffer from sleep apnea and its associated health risks. Further research and regulatory approvals are eagerly anticipated to determine the full potential of this novel treatment approach.Wikileaks Founder Julian Assange Pleads Guilty in U.S. CaseWikileaks Founder Julian Assange Pleads Guilty in U.S. Case Wikileaks founder Julian Assange has agreed to plead guilty to a felony charge related to his alleged role in the U.S. government’s largest breach of classified material. The plea deal, struck with the Justice Department, will allow Assange to avoid imprisonment in the United States. According to court documents, prosecutors will seek a 62-month sentence, which is equivalent to the time Assange has served in a high-security prison in London during his extradition battle. The plea deal would credit this time served, enabling Assange to return to his native Australia immediately. The plea deal requires approval from a federal judge. Assange initially faced 18 counts from a 2019 indictment for his alleged role in the breach, carrying a potential maximum penalty of 175 years in prison. U.S. authorities allege that Assange encouraged former army intelligence analyst Chelsea Manning to obtain classified military records, including diplomatic cables, Iraq War reports, and information about detainees at Guantanamo Bay. This information was published by Wikileaks in 2010 and 2011. President Biden has hinted at a possible deal with Australia to return Assange home. However, FBI and Justice Department officials oppose any agreement that does not include a felony guilty plea from Assange. Last month, a UK court ruled that Assange could appeal his challenge against extradition to the U.S. This was a victory for Assange in his ongoing fight against prosecution in the United States.
Diabetes Medication Shows Promise in Treating Sleep Apnea A new study has found that a medication used to treat diabetes may also be effective in resolving sleep apnea. The study, published in the journal *Chest*, found that the medication, liraglutide, reduced the severity of sleep apnea in up to 52% of patients. Sleep apnea is a condition in which breathing repeatedly stops and starts during sleep. This can lead to a number of health problems, including heart disease, stroke, and obesity. The current standard treatment for sleep apnea is continuous positive airway pressure (CPAP) therapy, which involves wearing a mask that delivers pressurized air to the throat during sleep. However, CPAP therapy can be uncomfortable and difficult to tolerate, and many patients do not adhere to the treatment. Liraglutide is a medication that is used to treat type 2 diabetes. It works by increasing the production of insulin, a hormone that helps the body use glucose for energy. In the new study, researchers found that liraglutide may also reduce the severity of sleep apnea by relaxing the muscles in the throat. The study involved 73 patients with sleep apnea who were randomly assigned to receive either liraglutide or a placebo. After 12 weeks, the patients who received liraglutide had a significant reduction in the severity of their sleep apnea, as measured by the apnea-hypopnea index (AHI). The AHI is a measure of the number of times per hour that breathing stops or becomes shallow during sleep. The researchers also found that liraglutide improved the patients’ sleep quality and reduced their daytime sleepiness. There were no serious side effects associated with liraglutide. The researchers say that their findings suggest that liraglutide may be a potential new treatment for sleep apnea. They are now planning a larger clinical trial to confirm their findings. If liraglutide is approved by the FDA for the treatment of sleep apnea, it would be the first medication to be approved for this condition.
Diabetes Medication Demonstrates Potential for Sleep Apnea Treatment
Related Posts
Kate Hudson Recreated Her Iconic How to Lose a Guy in 10 Days Scene During the World Series, and I Can’t Ignore the Fans’ Reaction to It
Kate Hudson isn’t just an award-winning one actress with famous parents; she is also a huge baseball fan. So it’s no surprise that she attended this year’s World Series to…
Software Catalog Unveils Array of Cutting-Edge Solutions for Enterprise Transformation
Software Catalog Unveils Array of Cutting-Edge Solutions for Enterprise TransformationSoftware Catalog Unveils Array of Cutting-Edge Solutions for Enterprise Transformation Technology is rapidly reshaping the business landscape, making it imperative for…